Theo Ross: 2024 Women in Biopharma R&D
Theo Ross, M.D., Ph.D. CPI’s, President and Chief Scientific Officer, was recognized as one of 20 women making an impact in Biopharma Research & Development in 2024 by Endpoints News. Dr. Ross serves as Vice President of Early Oncology R&D at AbbVie. Read about her journey from medical school to pharma and how empathy drives her work.
Conference BRCA 30 Years: Discovery to Impact
Nov 4-5, 2024 – Hybrid Event
The Women’s College Hospital (WCH) has organized a special hybrid conference to celebrate the 30-year anniversary of the discovery of BRCA genes. The conference will cover research and innovations related to BRCA genes and BRCA-related inherited cancers. Many prominent cancer researchers and oncologists, including CPI scientist Dr. Joanne Kotsopoulos, will present their work at this event.
We encourage you to attend this event in person or virtually to learn how the discovery of BRCA genes has influenced cancer research and cancer patients. DAY 1 caters mainly to researchers and healthcare providers and DAY 2 to patients and their families. Please see the links and documents below for more information.
Damon Runyon Fellowship Award
CPI has partnered with Damon Runyon Research Foundation to promote research relevant to the development of molecular approaches (such as drugs and vaccines) for the prevention of inherited cancers. If an applicable proposal is selected by Damon Runyon’s award selection committee and approved by the Damon Runyon Board of Directors, CPI will co-fund the Fellowship Award.
Award Overview – https://www.damonrunyon.org/for-scientists/application-guidelines/fellowship
Application Guidelines – https://www.damonrunyon.org/for-scientists/application-guidelines/fellowship/forms
Projects considered for funding by CPI are those that have the potential to make significant contributions to the development of chemo- or immuno-prevention measures that could reduce the risk of cancer in germline mutation carriers. Discovery research that could increase the understanding of early, pre-cancer stages are also considered. Visit the link below for more information on CPI’s funding priorities.
https://www.cancerpreventioninitiative.org/apply-for-grants/funding-priorities/
We encourage postdoctoral researchers with novel and innovative ideas related to precision prevention of inherited cancer to take advantage of this opportunity. Please contact Grants@CancerPreventionInitiative.org with questions regarding the CPI-funded award.
In Memory of Dr. Charis Eng
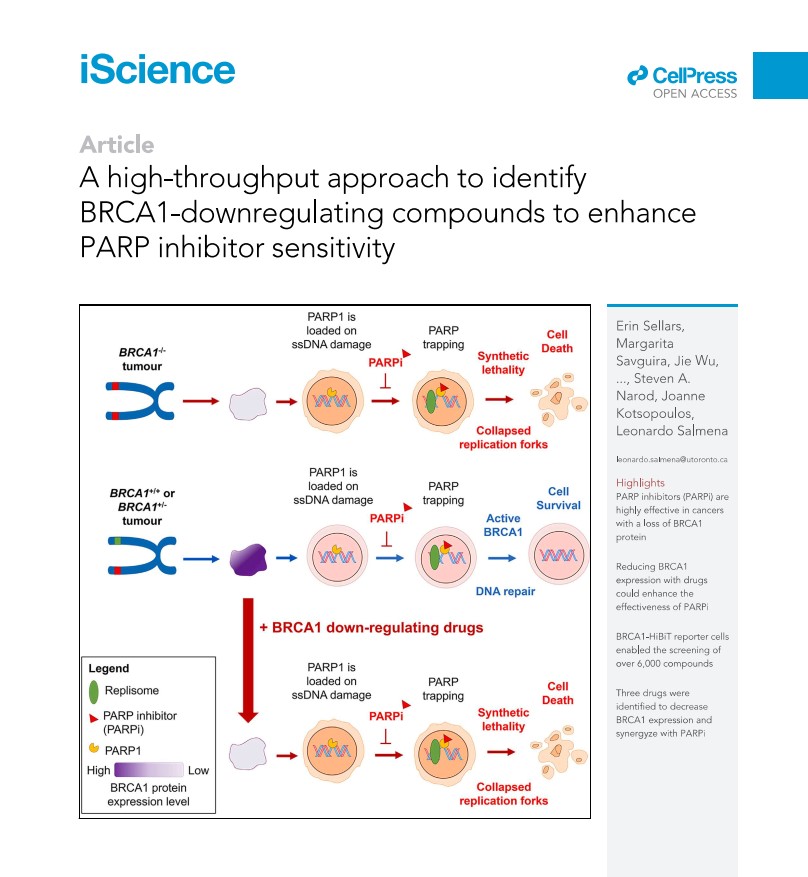
A high-throughput approach to identify BRCA1-downregulating compounds to enhance PARP inhibitor sensitivity
A CPI grant funded Dr. Kotsopoulos at the University of Toronto, Toronto, Canada for Evaluating BRCA1 haploinsufficiency as a putative target for the prevention of BRCA1-associated cancer. She and her collaborators developed a cell-based test to screen for drugs, small molecules, or natural compounds that could alter BRCA1 levels. Following the screening of more than 6,000 compounds using this assay, they identified several agents that can decrease BRCA1 protein levels. Some of these compounds can sensitize breast cancer cells to the PARP inhibitor Olaparib and may therefore have future clinical utility.
Read the full article in iScience.
Li Fraumeni Syndrome and one family’s harrowing experience with this deadly disease
Li Fraumeni (LF) is a hereditary cancer syndrome due to deleterious inherited mutations in the TP53 gene that increases the risk for developing a wide range of cancers, mostly sarcomas, breast cancers, brain tumors, and leukemias.
In the Wall Street Journal article below, journalist Lawrence Ingrassia writes about the multiple deaths in his family from a then unknown cause, and the scientists whose dedicated research spanning decades that eventually unearthed the culprit.
Much has been learned about LF since the death of the author’s mother in the 1950’s; when the author’s brother died after developing multiple cancers in 2019, he knew that he carried a TP53 mutation. However, the disease is still managed in mutation carriers by frequent screenings and tests to catch the cancers as early as possible. There is currently no way to prevent the disease before cancer(s) have taken hold.
As such, CPI funded a project by Dr. Jos Jonkers that could lead to vaccines that prevent cancers with TP53 mutations.
Solving the Cancer Mystery That Devastated My Family
For decades, Lawrence Ingrassia wondered why so many of his loved ones got cancer. Then a team of dedicated researchers discovered the gene p53.
By Lawrence Ingrassia
My most enduring childhood memories of my mom are of her being sick. Of visiting her in the hospital with my older brother and two younger sisters. Of our grandmother staying with us while our mom recuperated from breast cancer surgery. Of seeing her in bed at home with a soulful, sad look on her face.
She had been ill, sometimes gravely, off and on while I was growing up. I’m pretty sure that she first had cancer as early as 1958, when she was 32, though my memory is vague because I was only 6.
2023 CPI Research Meeting
The second Cancer Prevention Initiative (CPI) Research Meeting was held on January 18th, 2023. Following the success of the first meeting in 2021, this event was again held virtually and was moderated by Dr. Larry Brody, Director of the Division of Genomics at the National Human Genome Research Institute at the National Institutes of Health. He is a strong advocate for cancer prevention and has made pioneering discoveries of the genetic basis of breast cancer and the roles of the breast cancer genes BRCA1 and BRCA2.
CPI President and Chief Scientific Officer Dr. Theo Ross opened the meeting by welcoming the attendees and thanking Dr. Brody for moderating the event again this year. She reiterated the mission and the strategy of CPI, to accelerate the discovery and development of new medicines that prevent cancer by supporting research on the prevention of inherited cancers. She shared the good news that in addition to the progresses made in the scientific research front, CPI has successfully raised substantial funds to continue support of current projects as well to as start additional projects this year. She also spoke of new funding opportunities in the external cancer prevention front: concept proposals by the National Cancer Institute’s Division for Cancer Prevention in support of research related to immunoprevention and the discovery of natural compounds that may prevent cancer. This is a welcome change, as prevention research has historically received weak support from funding agencies. Even today, only a small fraction of the funding is devoted to prevention, and treatment remains the main focus. These new efforts, though small, may herald a rising interest in prevention by funding agencies.
The 2023 CPI research meeting featured a lineup of CPI-funded researchers who presented updates on their research projects. The first session of the meeting was dedicated to research projects on immunoprevention of cancer, harnessing the abilities of the immune system to stop precancerous lesions from developing into cancer. Dr. Peter Lee of the City of Hope Comprehensive Cancer Center discussed his project related to repurposing an FDA-approved antiparasitic drug that they showed could kill nascent tumor cells in a way that activates the immune surveillance system to prevent future cancer initiation.
The next presenter, Cleveland Clinic’s Dr. Charis Eng, started her presentation with a historical imperative for prevention from the ancient Chinese medical text Huang Dee Nai-Chang: “Superior doctors prevent the disease, mediocre doctors treat the disease before evident, and inferior doctors treat the full-blown disease”. This philosophy resonates with the mission and vision of CPI to shift the approach to cancer from treatment to prevention. Dr. Eng and her team are working on developing a vaccine that would specifically target BRCA1 mutated cells before they can proliferate into harmful tumors and can be given to BRCA1 mutation carriers to reduce their chance of developing cancer. Along the way, they have generated the first gene expression data comparing tumor and normal mammary tissue of BRCA1 mutation carriers. These data are an invaluable resource for all researchers studying breast cancer.
The second session was dedicated to projects exploring the earliest events that drive cancer initiation and understanding the mechanisms underlying these events. BRCA1 mutation carriers have only one “good” copy of BRCA1 instead of the normal two. It is well known that precancerous cells develop when the “good” copy of the BRCA1 gene is lost. Dr. Maria Jasin and her team at Memorial Sloan Kettering Cancer Center have generated a very elegant cell-based assay to identify compounds and signaling pathways that contribute to BRCA1 gene loss. Their work can lead to finding ways to intercept this early step necessary for transformation.
CPI-funded researchers at the Women’s College Research Center at University of Toronto, Drs. Joanne Kotsopoulos and Leonardo Salmena, and Ph.D. candidate Erin Sellars, attempt to prevent cancer development in BRCA1 mutation carriers by restoring BRCA1 expression levels to normal levels. They used a high-throughput screen they developed to identify drugs that increased BRCA1 expression, and they have hit upon some promising targets.
The CPI Research Meeting was an opportunity for CPI scientists to share their newest data and get productive feedback from fellow scientists. It was also an opportunity for cancer scientists with a shared interest in prevention to come together and have a larger conversation on the challenges of carrying out cancer prevention research – from limited funding and lack of enthusiasm from the medical community to restrictions posed by regulatory agencies. These are some of the challenges CPI attempts to address through its goals.
The meeting ended with closing remarks by Dr. Ross.
The meeting was supported by generous funding from Lyda Hill Philanthropies.
Moderator:
Lawrence Brody, Ph.D.
Director, Division of Genomics
National Human Genome Research Institute
National Institutes of Health
Participants:
Charis Eng, M.D., Ph.D. Presenter
Professor, Sondra J. and Stephen R. Hardis Endowed Chair in Cancer Genomic Medicine
Lerner Research Institute, Cleveland Clinic
“Transcriptome guided vaccine for BRCA1/2 germline mutation carriers”
Maria Jasin, Ph.D. Presenter
Professor, Lab Head
Memorial Sloan Kettering Cancer Center
“Preventing LOH in BRCA mutation carriers”
Joanne Kotsopoulos, Ph.D.
Scientist, Familial Breast Cancer Research Unit, Women’s College Research Institute
Associate Professor, Department of Pharmacology & Toxicology, University of Toronto
“Screening for modifiers of BRCA1 expression”
Peter P. Lee, M.D. Presenter
Chair, Department of Immuno-Oncology
Professor, Department of Hematology & Hematopoietic Cell Transplantation
City of Hope Comprehensive Cancer Center
“Chemo-immunoprevention for cancer via repurposing a low-cost, safe, anti-parasitic drug”
Steven Narod M.D., FRCPC, FRSC
Tier 1 Canada Research Chair in Breast Cancer, Women’s College Research Institute
Professor, Dalla Lana School of Public Health, University of Toronto
Ying Ni, Ph.D.
Assistant Professor, Cleveland Clinic Center for Immunotherapy and Precision Immuno-oncology
“Transcriptome guided vaccine for BRCA1/2 germline mutation carriers”
Leonardo Salmena, Ph.D.
Associate Professor, Department of Pharmacology and Toxicology, University of Toronto
Affiliate Scientist, Princess Margaret Cancer Centre
Canada Research Chair, Tier 2
“Screening for modifiers of BRCA1 expression”
Erin Sellars, M.Sc. Presenter
Ph.D. Candidate, Salmena Lab
Department of Pharmacology & Toxicology, University of Toronto
“Screening for modifiers of BRCA1 expression”
CPI team participants:
Doug Hager, Ph.D.
CPI Sr. Vice President, Project Management and Operations
Theo Ross, MD, Ph.D.
CPI President and Chief Scientific Officer
Marion Stewart-Thomas, M.S.
CPI Operations Manager
Angelique Whitehurst, PhD.
CPI Sr. Scientist and Advisor
Ranjula Wijayatunge, Ph.D.
CPI Project Manager
2023 CPI Research Meeting Summary
Expert Interview with Alan Venook, MD, on colorectal cancer and the promise of immunotherapy for prevention and treatment
Posted February 9, 2023 –

Dr. Alan Venook is a nationally renowned expert in gastrointestinal cancers such as colorectal cancer (CRC) and liver cancer. He is based at University of California, San Francisco (UCSF) and holds the Madden Family Distinguished Professorship in Medical Oncology and Translational Research. He is also the Shorenstein Associate Director for Program Development at UCSF’s Helen Diller Family Comprehensive Cancer Center. Dr. Venook has a deep interest in clinical trial designs for the treatment of gastrointestinal malignancies and served as the Chair of the Gastrointestinal Committee of the Alliance for Clinical Trials in Oncology.
Dr. Venook earned his bachelor’s degree from Rutgers University, and his medical degree from UCSF. He completed his residency in internal medicine at UC Davis. He joined the faculty of UCSF in 1988.
In this interview, Dr. Venook shares his thoughts on:
- His motivation to focus on gastrointestinal (GI) cancers
- A recent study that shows the promise of first-line immunotherapy for rectal cancer (Cercek et al., 2022. New England Journal of Medicine – NEJM) and its implications for future treatments and Lynch syndrome patients
- The relevance of the gut microbiome to CRC and cancer treatments
- The shifting demographic in colorectal cancer (CRC)
- Changes in screening recommendations
- How to be an informed patient
- His observations regarding the difference between right- and left-sided colon cancer
Dr. Venook, you specialize in gastrointestinal (GI) cancers and have been in the field for many years. What spurred your interest GI cancers and especially colorectal cancers (CRCs)?
I wanted to work in an area that was really underserved and underexplored, and GI cancer seemed like a good area. Frankly, it was a practical issue of where the opportunities were; finding an area that was open and had opportunities for me. Obviously, I find it very interesting. CRC, particularly, is unique in that it’s one of the very few cancers that can be cured even after it’s metastasized. We didn’t know that when I started and ours was one of the first groups to demonstrate it. We’ve learned a lot about the disease since I started. We have a lot of treatments for CRC now, but it’s a complex disease and we are still playing catch-up. It has been challenging especially with the limits of efficacy of immunotherapies.
On the topic of immunotherapy, what do you think about the recent study (Cercek et al., 2022, see summary below) that treated rectal cancer patients with neoadjuvent immunotherapy and saw complete remission in all patients?
This is the first study I have seen that shows complete remission in every single patient. Nothing’s ever hundred percent, so they must not have treated enough patients to see non-responders. Nonetheless, it’s remarkable that every single patient treated went into remission. It is also remarkable that there was no toxicity. I believe that this may be the only journal paper I’ve ever seen on a clinical trial that had no toxicity. Again, they probably didn’t treat enough patients.
Memorial Sloan Kettering (MSKCC) has sort of led the field in what’s called “total neoadjuvant therapy” (TNT) for rectal cancer. In TNT, chemotherapy and radiation is done up front before surgery. Memorial had looked at TNT in dMMR/MSI-high patients and found that a surprising number of patients didn’t get the full benefit of neoadjuvant chemotherapy, about one out of three did not (Cercek et al., 2020). Based on those results, it seemed logical to flip the paradigm and go with the immune checkpoint inhibitors (ICIs) upfront.
A previous study from the Netherlands (NICHE study) (Chalabi et al., 2020) had looked at immunotherapy in patients with primary colon, not rectal, cancers in a neoadjuvant setting – ICIs given as primary treatment. They took the patients to surgery six weeks in, so, they really didn’t get a definitive glance, but they still saw really marked responses.
How long do you think it takes for different ways of treating patients to be adapted into to mainstream care? With the low toxicity seen in this study by Cercek and others, there doesn’t seem to be a lot of harm in trying neoadjuvant ICI.
The short answer is that it depends. If it’s a subtle or nuanced difference, it can take a very long time. There’s harm if you’re not vigilant and miss opportunities. We have to follow the data, and when the data tells us to make changes, we should go ahead and make changes. But most oncologists don’t live and breathe one disease. Therefore they may go with what they’re used to, but it’s our job to be ready to turn on a dime.
A lot of cancer care is dictated by the National Comprehensive Cancer Network (NCCN) guidelines, rightly or wrongly. And I happen to be the vice chair of the NCCN panel for guidelines to colorectal cancer and this neoadjuvant immunotherapy will be in discussion. Do we change the guidelines? It’s all consensus. It’s unusual that a 12-15 patient study could change the standards of care, but I think this is one of the times where, in my opinion, it might. If you change the guidelines, you need to include the proper caveats about exactly what you have to do, the right follow up and surveillance, etc. You can’t be nonchalant about it.
A concern in moving ICI up front is that you may miss the opportunity with patients who don’t benefit from the ICI but may get substantial benefit from chemotherapy. I think that one of the problems with immunotherapy is that there is truly so much upside to immunotherapy that physicians don’t want to abandon it once they start it. Because they assume it’s going to work. They may start with a single agent and if it doesn’t work add in another. Then they may see so-called “Pseudoprogression.” I think there are more papers written about pseudoprogression than patients who’ve actually had pseudoprogression. It’s talked about a lot. The danger is that these patients may never get chemotherapy due to physician and patient bias, and that may disadvantage some patients who might benefit from chemotherapy.
Given the low toxicity, how feasible is it to use ICIs in primary prevention for high-risk individuals, such as Lynch syndrome patients who are at a high risk for developing CRC and other cancers?
It is an interesting question. The problem with the ICIs is that they could have major downsides. I have a patient who is a perfect candidate for everything else, got a single dose of the PD-1 blocker Pembrolizumab, an ICI, just one dose, and has essentially myasthenia gravis. The patient had to be intubated and has been in our rehab for four months. Myasthenia gravis is an autoimmune neurologic disease, which is admittedly very unusual, but it happens. Probably one in 25 patients on ICIs have a major consequence, therefore, going into healthy beings with that kind of risk is not tenable.
How good an indicator is the MMR/MSI status in predicting response to ICIs?
As seen in Keynote 177 (Andre et al., 2020; Diaz et al., 2022) and other studies, even dMMR/MSI-high colon cancer is not uniformly responsive to ICIs. However, in the recent Cercek et al., 2022 study, all dMMR/MSI-high rectal cancers responded. Why is that? There are many differences between the colon and the rectum. Memorial Sloan Kettering has collected a lot of biospecimens, so they’ll have a ton of molecular data and a real opportunity to figure out the differences.
Can you discuss the relevance of the gut microbiome to cancer therapy? What is your opinion on using the microbiome to predict treatment responses and direct treatments?
We’ve known that the gut microbiome influences treatment responses for a long time. One of the observations is with the conventional chemotherapy drug, Capecitabine, an oral chemotherapy drug that’s used frequently in patients with colon cancer. It was observed that patients in the US tolerate a much lower dose than patients in Europe. Now, we’ve got quite good evidence that that’s almost directly related to the microbiome. Capecitabine is a prodrug that has to be activated in a couple of steps by several enzymes in order to be effective. People can have biota with activating and/or inactivating enzymes. These enzymes can change how the drug is processed and can play havoc with the dosing and predictability of the drug.
The microbiome has a huge impact on the whole immune system. I think that’s why the immune therapies may not work well in colon cancer. It’s also probably why patients with liver metastases with MSI-high tumors are less likely to get benefits from ICI than those with other sites of metastasis. Because the liver is sort of an immune island.
The problem with the microbiome is that it is very complex. Until the last decade we didn’t have the computational power to even sort through it. It is said that there are more microbiota in the microbiome than there are cells in the human body. I don’t know who counted them, but that alludes to the complexity of the microbiome.
In the future, I expect the gut microbiome to be incorporated into detection and treatment of cancer. I won’t be in the field by then. Jim Allison won the Nobel Prize for ICIs that he’d been fiddling with for 10 or 15 years before he really put two and two together. Hopefully, we will figure out the microbiome and how it influences the immune system and immunotherapies without taking so long.
What other immunotherapy approaches apart from ICIs do you see as promising? Where does adoptive T cell therapy stand for CRC?
Ideally, you would want to generate a unique CAR-T (chimeric antigen receptor T-cell) for each patient. One challenge is that it takes several months to prepare the T-cells. And you need patients who are Olympic athletes, patients to be in very good condition. These patients will already be having standard therapies, and you hope that they don’t deteriorate while the T-cells are being prepared. There is also the issue of efficacy with this therapy. Another problem, with any therapy actually, is that the nature of the disease changes from the time you put a patient on the study to the time you actually get to treat them. This could be a very daunting experience. However, we do have a program at UCSF, and we’ve got some patients who’ve made it through.
What are the major challenges in CRC space right now?
The biggest issue right now is the preponderance of young people with CRC. For the last decade or so, we’ve been seeing an influx of young people with CRC. The incidence of CRC in people over the age 60 has gone down dramatically because of screening, but I would say that it’s made-up for by the increase in younger people. So, the net incidence is about the same.
A vast majority of cases are at advanced stages like stage 4, because colon cancer symptoms take a while to develop. We probably have two dozen patients in our care at UCSF who are under the age of 40 with advanced CRC. If someone happens to have a cancer that bleeds that is good luck because that could lead to early detection. Otherwise, the cancer can be there for years before its detected. Then, there are times when there are symptoms, but it doesn’t occur to the patient or the doctors that it could be CRC.
Is this why some recent recommendations for CRC screening have been lowered from age 50 to 45? Do you think we should make the age even younger?
Screening people starting at 45 is not going to improve early detection. I guess it’s better than starting at 50. But it’s not going to make a big impact because although there are some cases between the age of 45 and 50, for the most part, the real increase is in 30-to-35-year-olds. The problem with increased screening is that there aren’t enough gastroenterologists to do that. Also, when you do many colonoscopies, you’re going to increase the chance of complications.
We need to figure out a simple test that can be used to screen for CRC less invasively. For example, a test that would detect protein biomarkers in stool. Or oncRNAs (orphan noncoding RNAs) that are excreted by cancer cells. At a recent conference, I saw phenomenal data from assays that are so sensitive, they can find oncRNAs in the stool. They could be predictive of cancer and therefore can be used for screening and identifying those who then need to get a colonoscopy. But, if it’s too sensitive you’re going to be putting people through too many colonoscopies.
Factors that increase the risk in the general population have a worse impact on high-risk individuals, such as lynch patients with inherited risk. What are some of the reasons for increasing CRC among young adults that they should know about?
This increase of CRC in young individuals is really mind boggling. It doesn’t appear to have anything to do with heritable factors. Interestingly, it also does not appear to be due to the obesity epidemic. It’s not only people with bad diets or with fatty livers or metabolic syndromes; we also see otherwise healthy people. We even see patients who are marathoners or triathletes.
The other thing that’s even more distressing is the disproportionate impact it’s having on people of color. Recently, a famous African American actor died of colon cancer at age 43 – this is not an isolated incident.
Aside from screening to detect cancer early, what are (early) interventions or preventative measures that are available for high-risk individuals?
There is no preventive therapy so far. For people truly at high risk, like with familial polyposis, the only choice is to remove the colon. The problem is the rest of the bowels are at risk for polyps as well. These people will not infrequently succumb to small bowel or other problems.
You’ve run many clinical trials, what are some of the challenges in planning and running clinical trials?
One challenge is that large clinical trials can take a long time to complete. The CALGB/SWOG 80405 study was launched in 2004 and I presented the results at the American Association for Clinical Oncology (ASCO) meeting in 2014. It was an almost 20-year process with 2300 patients. So, you need to have patients and patience.
It is getting harder to recruit patients for large, randomized trials which is how you would like to ask a good scientific question and be vigorous. But patients will come already decided on what they want based on a relatives’ recommendation or an anecdote they’ve seen on social media. It is harder do a large trial with a control or placebo arm.
How can one be an informed patient?
You want informed patients, but you don’t want them to be poorly informed or misinformed. There’s so much misinformation on the internet and we are constantly battling with it. It’s a real challenge. To me, it’s interesting that people may know about pharmacogenetics, how an individual patient’s genes effect the way drugs are metabolized. A common chemotherapy for multiple types of cancer is Fluorouracil (5FU). There’s an enzyme responsible for metabolizing it called DPYD. Certain rare variants of this enzyme can put some people, maybe 2% or 3% of the population, at greater risk for complications. There’s an advocacy group of family members of people who had complications of 5FU who are pushing for everybody to be screened for variants in DPYD. If we test everyone, we will find high-risk variants, but we will mostly find variants that we have no idea what to do with. For patients with these variants, it will be unclear what to do. Do we reduce the dose and run the risk of having a reduced impact on the patient’s outcome?
Being informed also means understanding that there are a lot of unknowns. Doctors should be ready to explain to patients why they wouldn’t do a test, as opposed to more and more patients demanding tests and getting them done. You should only do a test if you’re prepared to do something with the results or know what to do with the results. I wrote an editorial a few months ago about a circulating tumor DNA (ctDNA) test (Venook et al., 2022). It was a blood test to find evidence that cancer was present in the body, but it couldn’t tell you where it was located, what you could do about it, etc. So, you are giving people a death notice with nothing to do about it.
You’ve demonstrated the difference in outcome between colon cancers developing in the right vs. left side of the large intestine. Can you explain the process of figuring this out?
We carried out the biggest colon cancer study in the US, the CALGB/SWOG 80405 study (Venook et al., 2017). Some aspects of our results were different from a study that was done contemporaneously in Europe. I was trying to figure out the biological reasons to how this difference could be explained, and we put right vs left location of tumor as something to think about. However, to simplify the data sheets, we had not included the location of the primary tumor. So, we had to look through 2400 charts to collect this information, which took a while.
Coincidentally, I was invited to give a memorial lecture for a colleague who had passed away. Looking through his publications in preparation for the lecture, I came across a very old study that basically looked at a bunch of therapies that we’ve long since abandoned. But in that paper, there was just a line that commented that patients with right sided primary lived for ten months while patients with left sided primary lived for fifteen months. This was many years ago when we were not nearly as effective with the therapies for colon cancer. I thought “wow” that’s a big difference. I’ve never heard that. I was stunned that nobody followed up on it. So, this motivated us to look into the sidedness of the tumors.
In our study, we saw a stunning 15 – 16-month difference in survival between left- and right-side patients. And genetically it makes sense because the right and left colon come from different embryonic structures – the right colon comes from the midgut and the left colon comes from the hind gut. We are trying to understand the molecular features that make the right vs left colon cancer different, but we don’t have an answer yet.
I love to talk about this observation that the right and left side cancers are different, for which I am credited for figuring out. But it’s embarrassing that I’ve been taking care of patients for all these years and hadn’t figured it out before. It’s very humbling.
Dr. Venook, it has been such a pleasure talking with you. Many thanks for sharing your insights.
Promise of immunotherapy for DNA repair deficient rectal cancer, Cercek et al. 2022 NEJM
Summary: This small but significant study establishes the efficacy of immunotherapy as first line/primary treatment for a type of DNA repair-deficient rectal cancer. The patients received a type of immunotherapy known as immune checkpoint inhibitors (ICIs). ICIs act by releasing the molecular breaks put on by cancer cells on the immune cells to stop the immune system from identifying and destroying them. The ICI given in this study was dostarlimab. It was given to the patients every three weeks for six months. This was different from standard practice in that immunotherapy was given as the first line treatment i.e., in a neoadjuvant setting before the main treatment. Surgery was planned for after immunotherapy. At the end of the six-month treatment cancer had disappeared in all 18 patients, and they avoided the need for surgery.
All the cancers in this study were deficient for a type of DNA repair called mismatch repair (MMR). Inability to repair errors in DNA due to deficiency in MMR (dMMR) results in high microsatellite instability (MSI-H) and the expression of cancer-specific proteins that help immune system identify the cancer cells.
This study is of particular interest to Lynch syndrome patients because they carry inherited mutations in MMR genes that put them at a high risk for CRC among other cancers. It raises the possibility of immunotherapy as first line therapy for Lynch patients with rectal cancer.
This study was conducted by Memorial Sloan Kettering Cancer Center (MSKCC) in New York with Dr. Luis A. Diaz Jr. as lead investigator.
Glossary:
Adoptive T cell therapy: A type of immunotherapy in which T cells (a type of immune cell) are given to a patient to help the body fight diseases, such as cancer. CAR-T is a type of adoptive T cell therapy, where the T cells are modified to better target and kill cancer cells before giving to the patient.
Familial adenomatous polyposis (FAP): A type of hereditary colorectal cancer, caused by pathogenic mutations in the APC gene. Many polyps (abnormal growths) form in the colon and the rectum and these may develop into cancer.
Immunotherapy: A type of treatment that uses a patient’s own immune system to fight cancer. Treatments may stimulate/activate the immune system to better identify and attack cancer. Includes checkpoint inhibitors, CART-cell therapy, cancer vaccines.
Immune Checkpoint inhibitor (ICI): A type of immunotherapy. Although the immune system can recognize many cancers and destroy them, the cancer cells develop ways to evade the immune system. They can produce “immune checkpoints” that can suppress the immune response, to put “breaks” on the immune response. Immune checkpoints are normally used to prevent autoimmune reactions against healthy cells of the body. ICI drugs like PD-1/PD-L1 and CTLA-4 inhibitors take these breaks off the immune system, to help it recognize and attack cancer cells.
Lynch syndrome: The most common inherited CRC syndrome caused by germline pathogenic variants in DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2) and EPCAM. Affected individuals have an elevated risk of developing CRC as well as certain other cancers.
Mismatch repair (MMR) and Microsatellite instability (MSI): Mismatch repair (MMR) is a type of DNA repair pathway that is present in the cell. MMR pathway components are encoded by genes such as (MLH1, MSH2, MSH6, and PMS2). MMR-deficient (dMMR) tumors have mutations in MMR genes and are thus unable to correct certain errors, resulting in tumors with high microsatellite instability (MSI-high) and mutational burden.
Neoadjuvant therapy: Treatment given first before the main treatment such as surgery.
Pseudoprogression: A phenomenon in which an initial increase in tumor size is observed, followed by a decrease in tumor burden. This phenomenon can benefit patients receiving immunotherapy but often leads to premature discontinuation of treatment owing to the false judgment of progression.
References:
Andre et al., 2020. NEJM. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer.
Cercek et al., 2020. Clin Cancer Res. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy.
Cercek et al., 2022. NEJM. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer.
Chalabi et al., 2020. Nat Med. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers.
Diaz et al., 2022. Lancet Oncol. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study.
Venook et al., 2017. JAMA. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial.
Venook et al., 2022. JAMA. Colorectal Cancer Surveillance with Circulating Tumor DNA Assay.
Expert Interview with David Reese, MD, on cancer prevention and precision medicine
Posted June 23, 2022 –
 Dr. David Reese is Executive Vice President, Research and Development (R&D) at Amgen. In his current role, he oversees Discovery Research, Global Development, Global Regulatory Affairs and Safety, and Global Medical. Prior to his position as head of R&D, he was the Senior Vice President of Translational Sciences and Oncology where he oversaw translation of Amgen’s drugs from the lab to the clinic and guided Amgen’s overall oncology strategy. He started his career as a clinical oncologist and was involved in a multitude of clinical trials that tested the safety and efficacy of cancer drugs, including trials for Herceptin/Trastuzumab, one of the first targeted therapies to be developed and which is used to treat certain types of metastatic breast and other cancers. He did his undergraduate studies at Harvard College and received his medical degree from the University of Cincinnati College of Medicine. In this interview, Dr. Reese shares his career path, views on the opportunities and challenges to developing cancer prevention drugs, and thoughts on precision medicine. This article represents Dr. Reese’s personal views as an individual and cannot be attributed to Amgen.
Dr. David Reese is Executive Vice President, Research and Development (R&D) at Amgen. In his current role, he oversees Discovery Research, Global Development, Global Regulatory Affairs and Safety, and Global Medical. Prior to his position as head of R&D, he was the Senior Vice President of Translational Sciences and Oncology where he oversaw translation of Amgen’s drugs from the lab to the clinic and guided Amgen’s overall oncology strategy. He started his career as a clinical oncologist and was involved in a multitude of clinical trials that tested the safety and efficacy of cancer drugs, including trials for Herceptin/Trastuzumab, one of the first targeted therapies to be developed and which is used to treat certain types of metastatic breast and other cancers. He did his undergraduate studies at Harvard College and received his medical degree from the University of Cincinnati College of Medicine. In this interview, Dr. Reese shares his career path, views on the opportunities and challenges to developing cancer prevention drugs, and thoughts on precision medicine. This article represents Dr. Reese’s personal views as an individual and cannot be attributed to Amgen.
Will you briefly describe to us your background and career path?
I am currently the head of research and development (R&D) at Amgen. I have been at Amgen for almost 17 years now. I was trained as a medical oncologist. After medical school, I did my training at University of California, Los Angeles (UCLA). I was a fellow and postdoc in the laboratory of Dennis Slamon, MD, PhD (1, 2) when the antibody that became Herceptin/Trastuzumab was going through preclinical work and then being developed. Following my tenure at the Slamon lab, I became a faculty member at UCLA, then at University of California, San Francisco (UCSF), and back at UCLA again, mostly doing translational research. My niche was early phase drug development. I moved to Amgen in 2005.
I have had a variety of roles in research and development at Amgen, initially running drug development programs in oncology, then the early oncology portfolio, then the entire early development portfolio across therapeutic areas – we focus on oncology, inflammation, and cardiometabolic disease. Then I ran what we call translational sciences, which had early development plus various other arms – toxicology, pharmacokinetics, metabolism, etc. I was part of the R&D leadership team, and then became head of R&D about four years ago. In my current role, I am responsible for crafting our research and development strategy and guiding those efforts.
Tell us a little more about the development of Herceptin/Trastuzumab.
HER-2 is a protein that is overexpressed, amplified, or, more rarely, mutated in a number of human cancers including breast, ovarian, and lung cancer. Overexpression or activation of HER-2 contributes to the increased proliferation and survival of cancer cells. At the Slamon lab, we were working on a mouse antibody (antibody 4D5) that could bind to HER-2 and block it, inhibiting its cancer promoting functions. This mouse antibody was later humanized and developed into Herceptin. UCLA was pivotal in the clinical trials and in addition to the pre-clinical studies, my colleagues performed the early combination trials for Herceptin/Trastuzumab as well.
In those days, platinum was not thought to be an effective agent against breast cancer. However, the team at Slamon lab had developed evidence that there was a synergistic interaction between Herceptin and platinum agents (3). Long story short, the effects of combinatorial treatment with Herceptin and chemotherapeutic agents were established in the phase III trial results in 1998 (4). Remarkably, a patient from this trial who had metastatic breast cancer and was treated with platinum plus Herceptin remained alive and in complete remission for many years.
Obviously, a large number of people worked on this over many years, and I had my very tiny little piece.
What are your thoughts on prevention or early interception of cancer?
The first question for me is in determining which tumors are clinically relevant within the context of early detection. Prostate cancer in a 90-year-old male is not something to intervene on, but acute leukemia is a different story and needs attention.
The second big issue is in detection. Do we have the technologies to detect tumors at the very early stages? There are several companies now sequencing circulating tumor DNA in the blood to detect tumors. Some of these tests are clearly detecting existing tumors. The technologies that allow for the early detection of cancer are coming. In the future, we are clearly going to screen for a full panel of cancers. On the molecular level, we can divide breast cancer into discrete diseases, and the same can be said for lymphoma and some other malignancies. Ultimately, where we want to get is to be able to screen using a blood-based assay for molecular signatures that can precisely detect or predict tumors. But we are still in the basic stage of this technology.
Right now, we’ve got a bunch of observational data from these tests. To me one real question with many of these data is in the validation of them. A really important question is, if you get a positive result, what does it mean? What if the imaging studies are negative, which they may well be because the number of cells may be well below the limit of detection. What do you do? How many of those patients with positive results will develop clinically evident cancer, and over what period of time? I don’t know whether there are any ways to answer these questions, except for doing very long longitudinal studies. I would love to hear of other approaches, but it is hard to get beyond the logic that you will need these big studies.
Are you optimistic that we are going to improve on current cancer detection methods?
That is another question: Are the new tests any better than the current methods? How do they compare with standard screening? But we’re not even equipped to answer that question yet. We need to take the first step before we take that second step.
You are taught as an intern, “Don’t order a test unless you’re going to do something with the results.” Because otherwise, you’re just going to create grief for everyone. So, for me this is a philosophical issue. If you are the “we have to do everything as early as possible and I want to know everything” kind of person, you are going to say “test.” If you are at the other end of the spectrum, you might say “I’ll do the standard stuff and I’ll wait to see how this technology evolves.” Given the gap in knowledge, these are both perfectly rational approaches right now. It’s no one’s fault, it is just the state of the art. To me we must address those big buckets; without that I don’t see a way forward.
We are collecting large amounts of observational data from these studies, and it is important. However, at a certain point these data are limited in what they can tell you. We need to prove that this technology is ultimately saving lives: that’s the real goal.
Do you think these initial tests, where they are gathering data, are more informative in a high-risk population, like one with inherited mutations in cancer predisposition genes, compared to the population at large?
If we have patients with an inherited predisposition, whatever it might be, BRCA1 or one of the many other mutations, now we have (by definition) identified a very high-risk population. The risk-benefit ratio in a population with inherited cancer gene mutations is very different to what I started with, which is a general population. With a high-risk population, we need to be more aggressive in our responses; if we pick something up, we’re going to do imaging every few months or other appropriate screening.
To me the high-risk populations are a proof of principle population in a way. It is an enriched trial population by definition. If the tests don’t work in the high-risk population, forget it, the chances of them working in the general population are low to none.
Where are we with the development of cancer prevention drugs? What is the interest in pharma?
Right now, that’s hard. Some of the the largest prevention studies to date (testing vitamins) gave us, in some instances, a contrary result in that those taking the preventive agent actually had higher rates of cancer. I think we will be much more keenly interested in prevention drugs once we feel that we have the right targets. We still have to solve many problems, such as accurately detecting cancer and validating and correctly interpreting the test results from detection tests.
What is your definition of precision medicine, and how can it influence cancer prevention and treatment?
From a drug developer’s perspective, precision medicine can be described by the simple phrase “the right drug, for the right patient, at the right time, and at the right dose.” It is obvious that oncology is where precision medicine has made the greatest inroads, because of molecular profiling of tumors and the advent of targeted therapies. I think we are just on the threshold of the era of human data that will lead to real precision medicine. It is part of our efforts to capitalize on the immense amounts of human data now available.
What do we mean by human data? At Amgen, we have sequencing data from hundreds of thousands of whole genomes. This is a huge increase from the couple of thousand we had a decade or so ago. But human data is not limited to genomics. We have genotypic data, combined with phenotypic data such as clinical information and demographic data, of two and a half million individuals. We also have total mRNA profiles (transcriptomics) and total protein profiles (proteomics) from tens to hundreds of thousands of individuals. So, this human data is not limited to genomics, it is multi-omic. This is more than 100 petabytes of data, even without including the real-life, clinical trial data we’ve got. This data will get richer in the future with biomarker work that will help predict disease progression and treatment outcomes.
The end game is not all these impressive amounts of data, but applying them to the individual patient, i.e., a true precision medicine. The folks who are able to ingest, aggregate, and critically analyze these large amounts of data are the ones who will push the field forward. We are in the early stages, but there are indications that this is coming fast. For example, it was known that the Artificial Intelligence (AI) company DeepMind was developing AlphaFold, a protein structure prediction program. But everybody thought it was still five or ten years off. Last July (2021), we went to bed one night and we woke up the next morning, and there were 350,000 predicted protein structures in a public database (5). The ability to predict the structure and function of a protein more quickly and efficiently using AI is an example showing that we are moving towards precision medicine very, very rapidly.
All this data and analysis will ultimately lead to precision medicine. I think it is sort of like the original motto of the United States “E Pluribus Unum”/ “from many, one,” meaning we will use the knowledge from large populations to help the individual patient.
What do you think are the biggest challenges in human data right now? Is it figuring out what to do with the data or is it figuring out how to cross-reference the data?
It is all the above. Of fundamental importance is the analytical engine. At a certain point the size of a dataset takes on a quality all its own, and we have reached that point and gone past that with our datasets. If you want to ship them somewhere, it takes two weeks over ultrafast pipes. It’s not like you pop up an excel spreadsheet. So, we have an enormously rich resource there.
It is also enormously dangerous. There is so much data that anyone with some smarts and a statistical package can find out a large number of correlations. What I always ask my team is, “which ones are true?” In genetics literature, a good fraction of papers published is reporting correlations, not true findings. It is similar to when microarray technology came out in the mid to late 90s. Everybody got a machine and all these papers came out. Many terrible papers came out because quality control was bad and the field had to clean itself up. I think we’re now at the moment in time with genetics data that, because of the ease with which you can do it, knowing what to do with it and how to correctly analyze it, is the challenge. It’s a smaller universe of folks that know how to do that accurately.
When it comes to cancer genomic data, aren’t most of what we have from late stage, malignant tumors? Aren’t genomic data from premalignant or early cancer stages still rare?
This is where I think some of the other technologies will be important because genomics in some ways is a starting point. We are looking at other areas such as cardiovascular disease, atherosclerotic cardiovascular disease. We have preliminary evidence, which I think almost certainly is going to pan out, that we can start to predict with a much higher degree of accuracy, which patients are going to have an event in the next few years based on changes in their proteome. Your genome is largely fixed. Your proteome varies over your lifetime. So, longitudinal sampling is critical here. What we’re interested in creating is a real precision medicine that can say, “hey, your proteome changed. We now know you’ve moved into a very high-risk category to have a myocardial infarction in the next two years, so aggressive intervention is warranted.” That’s where I think all of this is heading.
References:
- https://cancer.ucla.edu/research/find-become-a-member/meet-our-leadership/dennis-slamon-director-clinical-translational-research
- These reports detail preclinical studies that show that the combination of trastuzumab and chemotherapeutic agents have negative synergistic effects on HER-2 overexpressing cancer cells.
https://profiles.ucla.edu/dennis.slamon
- Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Pietras RJ, et al. Oncogene. 1994. PMID: 7911565
- Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Pegram MD, et al. Oncogene. 1999. PMID: 10327070
- Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. Pegram MD, et al. J Natl Cancer Inst. 2004. PMID: 15150302
- Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. Pegram MD, et al. J Natl Cancer Inst. PMID: 15150304
- Highly accurate protein structure prediction with AlphaFold. Jumper J, et al. Nature. 2021. PMID: 34265844
Expert Interview with Ray Deshaies, PhD, on drug development and cancer therapeutics
Posted on March 14, 2022 –
 Dr. Ray Deshaies is Senior Vice President of Global Research at Amgen and a Visiting Associate at the California Institute of Technology (Caltech).
Dr. Ray Deshaies is Senior Vice President of Global Research at Amgen and a Visiting Associate at the California Institute of Technology (Caltech).
Prior to joining Amgen, Dr. Deshaies received his BS in biochemistry from Cornell University, and his PhD in biochemistry from the University of California, Berkeley. He performed his postdoctoral research at UC Berkeley and the University of California, San Francisco. This training led him to start his own research group at Caltech studying how cells dispose of proteins to maintain homeostasis and how disrupted regulation of this process leads to disease, including cancer. Building on this academic work, Dr. Deshaies co-founded several biotech companies to commercialize technologies with therapeutic potential.
Dr. Deshaies received numerous awards for his outstanding research contributions. He has been elected as a Fellow of the American Association for the Advancement of Science (AAAS), as a Member of the American Academy of Arts and Sciences (AAA&S), and as a Member of the National Academy of Sciences (NAS). He was also selected as a Howard Hughes Medical Institute (HHMI) investigator.
In this interview, Dr. Deshaies explains the path to his current role at Amgen. He gives us the unique perspective of someone who has experience in both academia and industry. He also describes many of the opportunities and challenges in drug discovery and cancer prevention and the necessity of both academic and industrial efforts in bringing innovative new medicines to patients.
This article reports Dr. Deshaies’s personal views as an individual and cannot be attributed to Amgen.
Could you tell us what inspired you to go into biomedical sciences?
My interest in biology started with an interest in plants. I grew up in inner city Connecticut and we had this tiny little yard next to our house where my dad would grow tomatoes. I started helping him, and then I started growing plants myself. It was a hobby, but something I became quite interested in. On a family friend’s suggestion, I applied and got into Cornell because they had a good agriculture school. My prospective major was horticulture, but I also had to take classes in the basic sciences – chemistry, basic biology, and physics. I became interested in understanding biology at a molecular level. Subsequently, I changed my major to Biochemistry and focused my undergraduate and graduate studies in this area.
After a long tenure at Caltech you left academia to work in industry. You’ve had a very successful academic research career and had been recognized for your contributions. Can you tell us what influenced your decision to transition to industry?
Part of the motivation was that after 23 years at Caltech, I was ready to do something different. I had achieved many goals that I had set out at the beginning of my career. I had run my own lab, made scientific discoveries, published high impact papers, and mentored many graduate students and postdoctoral fellows. The prospect of learning new things in a different environment appealed to me.
While in academia, I had been involved with biotech start-ups and had some first-hand experience of the process of drug development in industry from an outsider’s perspective. I realized that by working in industry, I would gain an additional perspective of the drug development process. Translational research was gaining ascendency and industry seemed, for me, the right place to be.
Having experience in both academia and industry, what would you say are the similarities and differences between these two environments?
Generally, a major difference I see between academia and industry is that of perspective. In academia, the motivation is to understand nature and a very small portion of the work is translational, while in industry you are primarily oriented towards developing drugs for patients. While academia is about basic science discovery, industry also includes application of discoveries.
As someone who has made basic science discoveries that led to successful drugs, can you discuss the importance of basic science research for drug development? In your experience, how does basic research inform and shape the development of cancer therapeutics at drug companies?
One great example of basic science leading to therapeutics is of the development of proteasome inhibitors that are now used in a variety of cancers, most notably multiple myeloma, a type of blood cancer. The proteasome is a protein complex that destroys damaged or unwanted proteins from the cell. Fred Goldberg at Harvard University was exploring ways to control protein degradation because he had an interest in muscle wasting that happens (for example, in late-stage cancer patients) due to excess protein degradation by the proteasome. Alongside efforts in his academic lab, he founded the small biotech company, MyoGenics, to develop compounds that inhibit the proteasome. They developed a molecule that is used to treat multiple myeloma. Notably, these researchers did not set out to study the proteasome with the intention of developing a drug for multiple myeloma. The sensitivity of multiple myeloma cells to proteasome inhibitors was a fortuitous discovery. But we would never have proteasome inhibitor drugs if not for the fundamental research driven by the curiosity to learn how cells degrade proteins.
A second example is the development of PROTACS (Proteolysis Targeting Chimeras). While at Caltech, I collaborated with Craig Crews, a chemical biologist from Yale with a shared interest in protein degradation. We conceived the idea of utilizing the proteasome to remove harmful or unwanted proteins. We designed these new molecules – PROTACs that can be used to target specific proteins for degradation. Craig started a biotech company and has developed drug candidates that are now in clinical trials for treating breast and prostate cancer.
The technology to develop PROTACS incorporated an understanding of the basic biology of regulating protein destruction and an extensive knowledge of chemistry to design compounds that can be used as therapeutics. More than twenty years since its inception in an academic lab, PROTAC technology is now being applied by many drug discovery labs and is currently a major interest to major pharmaceutical companies for its applicability to various diseases, not just cancer. It has also spun off novel technologies to destroy proteins through other mechanisms rather than the proteasome and to target other types of molecules in the cell, such as RNA.
What are the major challenges in drug discovery space? Lack of a detailed and comprehensive understanding of the disease could be an obstacle; is the challenge in the basic biology? Or is it targeting efficacy?
I don’t think one challenge dominates over the other, but targeting is a major challenge. A vast majority of known disease-causing proteins are yet to be targeted pharmacologically. This continues to be a major problem in inhibiting the known drivers of cancer. In addition, the complexity of the biology remains a problem. One major challenge comes from the interconnection of signaling pathways that convey information from outside the cell to the inside. The different signaling pathways are not linear but are connected to each other like a web. You may target one component of a signaling pathway with a drug, but then a different component of the signaling web would step in to compensate. So, you need to understand the complexity of the whole signaling web to effectively design inhibitor approaches. You may need to use combinations of drugs to target multiple components to overcome redundancy or compensation.
What is important for our readers to know about the drug development process? Can we accelerate the process of transitioning basic discoveries into therapeutics?
Successfully transitioning a molecule from the bench to a drug for the patient is a costly and lengthy process. Usually, it takes several decades to take a discovery and develop it into a drug for a patient. Once a basic science discovery is made, industry takes the basic discovery and does the applied work of making a drug. To make a drug, thousands of compounds have to be evaluated. Making and testing these compounds is expensive, and the cost is well beyond the budget of academic labs. A range of expertise is required from multiple scientific disciplines including medicinal chemistry, pharmacology, drug metabolism, safety, pathology, etc. to ensure a therapy is safe and effective before testing in humans. Many steps in this process are still empirical and empirical is inherently slow.
Academia and industry both have critical roles to play in drug discovery. Academia is really good at asking open ended questions, figuring out mechanisms, and in-depth characterization. Industry is well set up to take fundamental observations and translate them into drugs. Academia develops the idea and demonstrates its initial feasibility, but the concept will die if not handed to the private sector, which can supply money and professional drug developers to provide additional insights and mature the initial ideas into a drug. Academia and industry are complementary, and they meet somewhere in the middle between discovery and application.
What advice would you give young researchers?
Looking back at my own career in research, one thing I always tried to do is avoid the hot research despite its appeal. With many people working on the same question, it becomes a race to make the next discovery. For me, this race took away from the fundamental joy of discovery. I’d rather work on a problem where I would be rewarded for my creativity and insight instead of speed.
As a graduate student, I had the opportunity to work on a fundamental question in protein transport, but a question only a couple of other labs were working on. I took a different approach from others and successfully identified the key component necessary for this process. This finding is now taught in every cell biology course everywhere. This early experience showed me that one does not have to work in the hottest field to make important discoveries.
Perhaps this notion can best be stated by a conversation I had once with Seymour Benzer, a senior scientist at Caltech when I started my lab. He expressed his interest in pit vipers. In particular, how pit vipers sense small temperature changes even at sizeable distances. This is how a pit viper can sense the presence of warm-blooded prey nearby. This puzzle was not on my radar and out of left field. Maybe I should have taken his advice, as years later, the Nobel prize was won by scientists who described the temperature sensory system! So, my advice is to ask a fundamental question about science that interests you and delve into it.
